Views: 222 Author: Rebecca Publish Time: 2025-12-10 Origin: Site











Content Menu
● Understanding Pharmaceutical Preparation
● Core Stages of Pharmaceutical Preparation Manufacturing
>> 1. Raw Material Preparation
>> 2. Purified Water and Utilities
>> 3. Pure Steam Generation and Sterilization
>> 4. Distillation and Water for Injection (WFI)
>> 5. Solution Preparation and Mixing
>> 6. Filling, Sealing, and Sterilization
>> 7. Inspection, Labeling, and Packaging
● Cleanroom Environment and Contamination Control
● Advanced Technologies in Pharmaceutical Preparation
>> Automation and Control Systems
>> Validation and Qualification
>> Data Integrity and Regulatory Compliance
>> Integration with Factory Design
● The Role of Utilities in Pharmaceutical Preparation
● Everheal's Contribution to the Industry
● Innovation and Future Trends in Pharmaceutical Preparation
>> Digitalization and Smart Factories
>> Advanced Sterilization Technologies
● FAQ
>> 1. What is pharmaceutical preparation?
>> 2. What is the difference between purified water and Water for Injection (WFI)?
>> 3. Why is sterilization critical in pharmaceutical preparation manufacturing?
>> 4. How do GMP guidelines affect pharmaceutical preparation?
>> 5. How does Everheal assist pharmaceutical manufacturers?
Pharmaceutical preparation manufacturing represents one of the most sophisticated and tightly regulated branches of modern industry. It encompasses the science, engineering, and management of transforming chemical or biological substances into effective, safe, and stable medicinal products suitable for human or veterinary use.
For Everheal, a leading Chinese company specializing in pharmaceutical equipment and integrated solutions, understanding and optimizing every stage of pharmaceutical preparation is fundamental. The company provides advanced systems such as purified water preparation units, pure steam generators, multi-effect distilled water machines, aseptic filling and sealing systems, and sterilization equipment that meet Good Manufacturing Practice (GMP) and international standards. This article explores in detail what pharmaceutical preparation manufacturing is, how it works, why it matters, and how companies like Everheal support global pharmaceutical plants in their pursuit of excellence.

The term pharmaceutical preparation refers to the complete process of creating medical products in usable forms—such as tablets, capsules, ointments, injections, or oral solutions—from raw materials and active ingredients. It covers multiple interconnected phases: formulation, processing, purification, sterilization, filling, and packaging.
The core mission of pharmaceutical preparation is to ensure each product is safe, effective, stable, and consistent. To achieve this, manufacturers must adhere to precise technical procedures and rigorous documentation standards. Every process, from the preparation of purified water to the final packaging of a sterile injection, directly impacts drug quality and patient outcomes.
Pharmaceutical preparation is not merely mechanical production; it is a combination of chemistry, microbiology, and industrial engineering, coordinated within a validated GMP environment. It requires high-precision equipment, skilled operators, and well-designed facility layouts that minimize contamination risks and streamline workflow efficiency.
A successful pharmaceutical preparation process generally includes several major stages. Each one builds upon the previous step to produce a final product that meets quality and safety requirements.
All pharmaceutical manufacturing begins with raw materials, including active pharmaceutical ingredients (APIs) and excipients. These components form the foundation of a drug formulation. Each material is verified for purity, safety, and consistency through analytical testing before use. Materials are stored in controlled environments to prevent degradation and cross-contamination.
One of the most critical aspects of any pharmaceutical preparation process is the quality of water used. Water acts as a solvent, cleaning agent, and even a component of many formulations. Everheal's advanced Purified Water Preparation Systems utilize reverse osmosis, electrodeionization, and ultraviolet sterilization to produce ultra-clean water.
This purified water meets stringent pharmacopoeia standards, ensuring that no ions, bacteria, or endotoxins are present. It is used extensively in solution preparation, equipment washing, and cleaning of containers and piping systems.
Pure steam serves as a fundamental utility for sterilization within the pharmaceutical environment. Everheal's Pure Steam Generators produce steam that is completely free of contaminants, making it ideal for sterilizing production equipment, reactors, pipelines, and storage tanks. The absence of non-condensable gases and impurities ensures sterilization integrity and system reliability.
For parenteral or injectable drugs, Water for Injection (WFI) is the highest grade of water required. Everheal's Multi-Effect Distilled Water Machines employ a multi-stage evaporation and condensation process to produce WFI that complies with global pharmacopeia standards. The distillation process removes microorganisms, endotoxins, and chemical residues to yield water of exceptional purity and stability.
Once utilities are prepared, the next step is formulation. Active components are dissolved or suspended in solvent media under controlled conditions. Mixing tanks, vacuum homogenizers, and stirring vessels ensure uniform distribution and precise concentration of ingredients. Process parameters such as temperature and pressure are monitored continuously to maintain chemical integrity and reproducibility across batches.
For sterile pharmaceuticals, precision filling and sealing are crucial. Everheal's Liquid Filling and Sealing Machines operate under aseptic conditions within classified cleanroom zones (commonly ISO Class 5 or Grade A environments). The equipment ensures tight control of filling volume, sealing integrity, and product sterility. After filling, containers undergo sterilization using steam autoclaves or dry-heat sterilizers, confirming product safety before labeling and packaging.
Quality inspection marks the final stage of pharmaceutical preparation manufacturing. Automated visual inspection systems detect particulate matter, improper seals, or cosmetic defects. Batch coding, serialization, and tamper-proof labeling safeguard product traceability. Packages are designed to protect against light, moisture, and mechanical damage during transportation and storage.
Pharmaceutical preparation manufacturing demands an exceptionally clean and controlled production environment. Airborne particles, microorganisms, and humidity fluctuations can compromise product quality. To prevent contamination, manufacturing takes place in cleanrooms equipped with High-Efficiency Particulate Air (HEPA) filtration systems, pressure differentials, and constant environmental monitoring.
Personnel movement, material transfer, and equipment cleaning follow strictly validated Standard Operating Procedures (SOPs). Everheal's systems are designed for contamination control; their polished stainless-steel surfaces and sanitary connections eliminate microbial adhesion and simplify cleaning and sterilization.
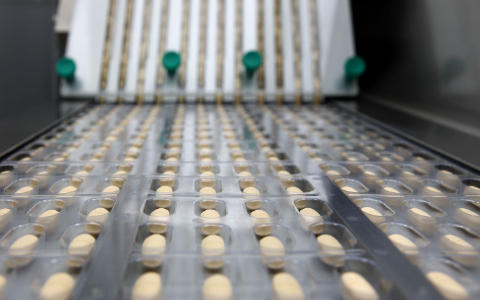
Modern pharmaceutical manufacturing integrates automation, digital monitoring, and quality assurance to maintain precision and efficiency.
Advanced automation reduces the possibility of human error and ensures repeatability. Programmable Logic Controllers (PLC) and Supervisory Control and Data Acquisition (SCADA) systems monitor every process parameter—from flow and pressure to temperature and pH—creating a stable and traceable manufacturing environment.
Before any equipment or process can be used in pharmaceutical preparation, it must undergo Installation Qualification (IQ), Operational Qualification (OQ), and Performance Qualification (PQ). These steps verify that systems are installed correctly, function as intended, and produce results that meet established quality specifications.
Digital recordkeeping under laws such as FDA 21 CFR Part 11 ensures data authenticity and traceability. Real-time monitoring, alarm systems, and electronic signatures guarantee that all operations are auditable and compliant with international GMP requirements.
Everheal not only provides individual equipment but also assists clients in complete factory design and layout optimization. The proper arrangement of production areas, air-handling units, and material pathways minimizes cross-contamination and enhances operational flow, aligning with EU GMP Annex 1 principles.
Utilities form the backbone of pharmaceutical preparation manufacturing. Without reliable supplies of purified water, pure steam, compressed air, and clean gases, consistent drug quality would be impossible.
- Purified Water Systems: Generate water for solution preparation, equipment cleaning, and washing.
- Water for Injection Systems: Provide ultra-high purity water for sterile formulations.
- Pure Steam Generators: Sterilize systems, pipelines, and production equipment.
- Clean Air Systems (HVAC): Maintain particle-free zones and environmental stability.
Each utility is subject to strict periodic maintenance, performance verification, and microbiological testing to ensure long-term reliability.
Pharmaceutical preparation manufacturing involves continuous monitoring at every stage. Samples are tested to verify concentration, pH, viscosity, and microbial content. Analytical instruments such as high-performance liquid chromatography (HPLC) and gas chromatography (GC) validate chemical purity, while biological assays confirm the absence of microorganisms or endotoxins.
Comprehensive quality documentation ensures traceability from raw materials to final products. Quality assurance systems cover auditing, deviation management, and change control, establishing transparency across all manufacturing steps.
Pharmaceutical preparation must comply with a complex web of regional and international standards. These organizations and regulations govern everything from raw materials to finished goods:
- FDA (United States Food and Drug Administration)
- EMA (European Medicines Agency)
- WHO GMP (World Health Organization)
- NMPA (China National Medical Products Administration)
GMP guidelines require companies to document every action, regularly validate their production processes, and maintain continuous training for operating personnel. Everheal designs its systems to be fully compatible with these international regulations, helping customers worldwide meet certification requirements swiftly.
As a specialized equipment manufacturer, Everheal delivers comprehensive, customized solutions for pharmaceutical preparation manufacturing. Its internationally certified product portfolio includes:
- Purified water preparation systems
- Pure steam generators
- Multi-effect distilled water machines
- Liquid filling and sealing systems
- Sterilization and washing equipment
- Cleanroom utility integration
By offering turnkey services—from conceptual design to final validation—Everheal empowers pharmaceutical manufacturers to build efficient, compliant, and future-ready production lines. Its technical teams collaborate with clients globally, developing complete factory layouts, system automation strategies, and maintenance frameworks ensuring long-term reliability.
The landscape of pharmaceutical preparation manufacturing is evolving rapidly. Advanced technologies and sustainability objectives are shaping the factories of tomorrow.
Instead of discrete batch production, continuous systems enable uninterrupted material flow, reducing downtime and human intervention. The result is improved efficiency, higher productivity, and fewer quality fluctuations.
Digital transformation allows predictive maintenance and real-time process optimization using Industrial Internet of Things (IIoT) platforms. Data analytics and machine learning can identify potential deviations before they impact product quality.
With environmental sustainability becoming a global priority, manufacturers now emphasize energy recovery, waste reduction, and water recycling. Everheal designs its systems with low energy consumption and high recovery efficiency, aligning with green manufacturing philosophies.
Innovative sterilization methods such as vaporized hydrogen peroxide (VHP) and plasma sterilization are gaining popularity. These methods provide high sterilization efficiency while leaving no harmful residues on equipment surfaces.
Modular construction enables faster deployment of pharmaceutical plants. Preassembled cleanroom units, utilities, and piping modules simplify installation and reduce project timeframes.
Through integration of these advancements, pharmaceutical preparation manufacturing is becoming smarter, safer, and more sustainable.
Pharmaceutical preparation manufacturing lies at the intersection of science, technology, and regulatory rigor. It ensures that every medicine reaching patients worldwide is produced under safe, controlled, and validated conditions. The process encompasses numerous steps—from purified water generation and material formulation to sterile filling and packaging—all designed to preserve safety and efficacy.
For companies like Everheal, pharmaceutical preparation is more than manufacturing; it's a mission to enhance global health through innovation and precision. With advanced engineering, GMP-compliant design, and global project experience, Everheal continues to empower pharmaceutical producers to achieve excellence in both quality and efficiency.

Pharmaceutical preparation is the process of formulating and manufacturing medicinal products in specific dosage forms, ensuring safety, stability, and compliance with health regulations.
Purified water is used for non-sterile processes such as cleaning or oral solutions, while Water for Injection undergoes distillation and is suitable for sterile pharmaceutical products like injections.
Sterilization removes or destroys microorganisms that could compromise drug safety. It ensures that sterile products remain contamination-free throughout their lifespan.
GMP ensures that products are consistently produced to quality standards by controlling equipment design, process validation, documentation, and staff training.
Everheal provides complete pharmaceutical preparation systems, cleanroom layouts, and turnkey project design, ensuring compliance with international GMP standards and reliable long-term operation.
Blueair purifiers use HEPASilent™ filtration technology to deliver HEPA Air Filter performance with enhanced quietness, energy efficiency, and higher particle capture. Learn how Blueair ensures cleaner air for households, offices, and even pharmaceutical-grade environments.
Discover whether you need a HEPA Air Filter for your air purifier. Learn how HEPA filters work, their importance in homes and pharmaceutical industries, and how Everheal’s advanced purification systems use HEPA technology to ensure safe, clean, and compliant air quality worldwide.
Learn how HEPA Air Filters work and why they’re the gold standard in air purification. Explore their mechanisms, applications in pharmaceuticals, hospitals, and homes, along with benefits, limitations, and maintenance tips for cleaner, healthier environments.
This article explores whether you can wash a HEPA filter air purifier, explaining the risks of washing True HEPA filters, how to maintain them properly, and how Everheal’s pharmaceutical systems integrate reliable HEPA air filtration for guaranteed purity and performance.
Maintaining HEPA air filters is critical for ensuring the highest air quality standards in pharmaceutical and manufacturing environments. Although it might be tempting to wash or reuse HEPA filters to save costs, doing so can compromise their integrity and filtration capability. Proper handling, timely replacement, and preventive maintenance practices are the best ways to sustain effective filtration. Always adhere to manufacturer guidelines and industry standards to guarantee a safe and contaminant-free environment.
In the modern industrial and residential air filtration market, the demand for sustainable, cost-effective, and high-performance filtration solutions has grown tremendously. Among the various options available, the washable air filter has become one of the most talked-about innovations. But what exactly makes it the best choice? How does it compare with disposable filters? And which washable air filter models stand out for efficiency and durability?
Discover whether a washable air filter is a good choice for your home or industrial system. Learn its benefits, maintenance tips, and limitations, and explore how washable air filters support sustainable air management in pharmaceutical and manufacturing environments.
Discover how air purifiers with washable filters improve air quality while cutting costs and environmental impact. Learn their benefits, applications, maintenance tips, and how they support sustainable manufacturing in homes, industries, and pharmaceutical facilities.
K&N washable air filters are durable, eco-friendly, and designed for lifetime use. Learn how to clean, maintain, and maximize performance from your washable air filter while reducing waste and boosting efficiency across automotive and industrial systems.
This detailed guide explains whether air purifier filters are washable, exploring how washable air filters work, their pros and cons, cleaning methods, and ideal applications in homes and industries like pharmaceuticals—helping users achieve efficient and sustainable air purification.
Everheal explains what pharmaceutical preparations are, their processes, and essential equipment like purified water systems, pure steam generators, and sterilization units. Learn how Everheal supports global manufacturers with GMP-compliant turnkey pharmaceutical solutions.
Everheal explores the engineering and technology of **pharmaceutical preparation manufacturing**, revealing how purified water, sterilization, and automation drive global drug production. Discover Everheal’s role in designing compliant, efficient, and sustainable pharmaceutical manufacturing solutions.
This article explores which pharmaceutical preparations need isotonicity adjustment, explaining its physiological importance, calculation methods, and the role of advanced equipment — such as Everheal’s integrated systems — in achieving safe, effective, and GMP-compliant drug formulations worldwide.
This article explains isotonicity in pharmaceutical preparations, its role in injections, eye drops, nasal sprays, and other dosage forms, and how advanced equipment systems like purified water and sterilization units ensure consistent isotonic conditions in pharma manufacturing.
Explore the different Pharmaceutical Preparations containing ammonium bromide, including their uses, manufacturing methods, safety measures, and modern significance. Learn how cutting-edge companies like Everheal ensure high purity and quality in pharmaceutical production.
This article explains why distilled water is strongly recommended for CPAP humidifiers, how it protects both users and equipment, and what happens when other water types are used. It also shows how Everheal’s pharmaceutical‑grade Distillation Water Machine solutions supply reliable CPAP‑grade water for homes, clinics, and hospitals.
This article explains when and how distilled water can be used with fog machines, from standard stage foggers to ultrasonic and low‑lying systems. It shows why distilled water is ideal for cleaning and certain water‑fog devices, and how a high‑purity Distillation Water Machine supports reliable, low‑maintenance fog effects in professional and pharmaceutical environments.
This in‑depth article explains why distilled water is strongly recommended for CPAP humidifiers, what happens if you use tap, bottled, or boiled water, and how to manage water safely at home, in clinics, and in pharma plants. It highlights how Distillation Water Machine systems provide reliable CPAP‑grade water.
This in‑depth guide explains why distilled water is strongly recommended for oxygen machine humidifiers, how it protects both patients and equipment, and how a Distillation Water Machine supports safe oxygen therapy in hospitals, clinics, and pharmaceutical plants, with Everheal turnkey solutions.
This article explains why distilled water is strongly recommended for CPAP machines, how it protects users and devices, and what happens if other water types are used. It highlights the role of pharmaceutical‑grade Distillation Water Machine solutions—like Everheal’s—in delivering reliable CPAP‑grade water for homes, clinics, and factories.