Views: 222 Author: Rebecca Publish Time: 2025-12-10 Origin: Site











Content Menu
● Understanding Pharmaceutical Preparations
● The Importance of Pharmaceutical Preparations
● Core Processes in Pharmaceutical Preparation
>> 1. Raw Material Handling and Quality Control
>> 2. Water Systems for Pharmaceutical Production
>> 3. Mixing, Blending, and Granulation
>> 4. Sterilization and Aseptic Processing
● Equipment in Modern Pharmaceutical Preparation
● Quality Assurance in Pharmaceutical Preparation
● Regulatory Requirements for Pharmaceutical Preparation
● The Role of Innovation in Pharmaceutical Preparation
● Benefits of Partnering with Everheal
● FAQ on Pharmaceutical Preparations
>> 1. What is meant by pharmaceutical preparation?
>> 2. What equipment is essential for pharmaceutical preparations?
>> 3. Why is water quality important in pharmaceutical preparation?
>> 4. How does sterilization affect pharmaceutical preparation?
>> 5. How does Everheal support international pharmaceutical manufacturers?
Pharmaceutical preparation is the foundation of modern medicine manufacturing – it encompasses the processes, systems, and technologies involved in transforming raw materials into safe, effective, and high-quality medications. For companies in the pharmaceutical industry, understanding the science and engineering behind pharmaceutical preparations is essential to ensure product safety, quality compliance, and efficiency.
As a leading Chinese manufacturer, Everheal specializes in providing complete pharmaceutical preparation solutions, including purified water systems, pure steam generators, multi-effect distillation units, liquid filling and sealing machines, and sterilization systems. By combining equipment innovation with design expertise, we help global pharmaceutical companies build advanced and compliant production lines.

Pharmaceutical preparations refer to drugs or medicinal products formulated and processed for therapeutic use. The process involves careful control of material selection, equipment sterilization, and quality management to maintain product purity and stability. In essence, pharmaceutical preparation is both an art and a science – one that integrates chemistry, engineering, and regulatory compliance.
According to the World Health Organization (WHO), a pharmaceutical preparation can exist in several forms: tablets, capsules, liquids, injections, ointments, and more. The composition of each product is determined by its therapeutic purpose, mode of administration, and shelf-life requirements.
The significance of pharmaceutical preparations lies in their ability to ensure consistent product quality, safety, and therapeutic efficacy. Every step—from material handling to final packaging—affects the medication's reliability. A single deviation in sterile conditions or contamination control can compromise the entire batch.
Key objectives of pharmaceutical preparation include:
- Maintaining purity from raw materials to finished product.
- Ensuring dosage uniformity and bioavailability.
- Preventing microbial contamination through classified cleanrooms and sterilized systems.
- Meeting Good Manufacturing Practice (GMP) and international standards.
In global pharmaceutical manufacturing, stringent regulations such as FDA (U.S.), EMA (Europe), and ICH guidelines govern every aspect of pharmaceutical preparation. Compliance is not optional—it is a necessity for product approval and market access.
Pharmaceutical preparation is a broad field covering numerous stages. Each phase requires precision, documentation, and specialized equipment.
The foundation of pharmaceutical preparation begins with high-quality raw materials. These include active pharmaceutical ingredients (APIs), excipients, and solvents. Every material must pass identification and purity tests before entering production. Automated weighing and dispensing systems minimize cross-contamination and ensure accuracy.
Water plays a critical role in pharmaceutical preparation. It serves as a solvent, cleaning agent, and raw material for many drug formulations. Everheal provides purified water systems and pure steam generators, both designed to produce water that meets the stringent requirements of pharmacopeias such as USP, EP, and JP.
Types of water used in pharmaceutical preparation:
- Purified Water (PW): Used for non-parenteral formulations and cleaning processes.
- Water for Injection (WFI): Produced through multi-effect distillation, primarily for injectable drugs.
- Pure Steam: Utilized for sterilizing equipment and pipelines in aseptic areas.
For solid dosage forms such as tablets or capsules, the pharmaceutical preparation process involves blending APIs and excipients to ensure uniform distribution. Wet and dry granulation techniques improve compressibility and content uniformity. Advanced mixing equipment reduces human handling, minimizing contamination risks.
Sterilization ensures that pharmaceutical preparations remain free from harmful microorganisms. Everheal's sterilization systems utilize steam, dry heat, and gas sterilization technologies according to specific product needs. For injectable or ophthalmic drugs, aseptic processing occurs in controlled cleanrooms where filtered air and pressure differentials maintain sterile conditions.
Liquid and injectable pharmaceutical preparations require precise filling and sealing operations. Fully automated liquid filling and sealing machines from Everheal achieve high-speed accuracy while minimizing product loss. These systems are engineered to comply with GMP standards and integrate features like in-line inspection and PLC control.
Packaging protects pharmaceutical products from environmental factors and ensures traceability. Blister packs, ampoules, vials, and bottles undergo inspection for integrity. Serialization and labeling systems add unique identifiers to prevent counterfeiting and comply with global supply chain regulations.
Cutting-edge equipment enhances efficiency, reduces contamination, and supports compliance. Everheal offers a complete range of advanced pharmaceutical preparation equipment suited for different formulations.
- Purified Water Preparation Systems: Provide continuous, validated water purity for formulation and cleaning.
- Pure Steam Generators: Produce high-quality steam for sterilization with energy-saving designs.
- Multi-Effect Distillation Systems: Generate Water for Injection using multiple stages of vaporization and condensation.
- Liquid Filling and Sealing Machines: Support precise dosing for ampoules, vials, and plastic bottles.
- Sterilization Systems: Ensure reliable sterilization cycles—vital for aseptic manufacturing environments.
Each piece of equipment is customizable based on production capacity, layout constraints, and regulatory requirements. Everheal's engineering team also designs complete factory layout plans and turnkey project solutions for global pharmaceutical facilities.

Quality assurance (QA) ensures that every product consistently meets established specifications. In pharmaceutical preparation, QA is implemented through:
- Standard Operating Procedures (SOPs): Define every manufacturing and cleaning step.
- Environmental Monitoring: Constantly tracks microbial counts, temperature, humidity, and particulates.
- Validation and Qualification: Confirms that equipment and processes operate as intended.
- Batch Record Review: Verifies every production log before product release.
- Corrective and Preventive Actions (CAPA): Identify and mitigate root causes of deviations.
By maintaining a strong QA system, manufacturers protect patient safety and uphold global trust in their products.
To sell pharmaceutical preparations internationally, manufacturers must adhere to global quality frameworks:
- Good Manufacturing Practice (GMP): Defines minimum requirements for production, environment, and documentation.
- ISO Standards: Ensure system-level quality consistency and risk management.
- FDA and EMA Guidelines: Govern approval of facilities and product validation in the U.S. and Europe.
- Chinese GMP and PIC/S Compliance: Needed for global export readiness and harmonized standards.
Everheal's equipment is designed to help manufacturers meet these compliance challenges. Each system undergoes Factory Acceptance Testing (FAT) and Site Acceptance Testing (SAT) before commissioning.
Innovation drives the evolution of pharmaceutical preparation. Modern facilities adopt automation, smart control systems, and cleanroom robotics to enhance efficiency and data integrity. Digital monitoring systems record all production parameters, providing traceability and regulatory transparency.
Everheal integrates intelligent control platforms that monitor temperature, pressure, and water quality in real time. Remote diagnostics and predictive maintenance minimize downtime while maximizing productivity.
Sustainability is another crucial innovation area. Energy-efficient steam generation systems and closed-loop water recovery technologies reduce waste and operational costs—making sustainable pharmaceutical production a realistic target.
Choosing Everheal means partnering with a team dedicated to technological excellence and compliance assurance. Our pharmaceutical preparation solutions provide multiple advantages:
- Turnkey project design and factory layout planning.
- Customizable equipment that meets global GMP standards.
- Energy-efficient and sustainable systems.
- Professional installation, validation, and training services.
- Reliable after-sales support and spare parts supply.
Whether building a new pharmaceutical facility or upgrading existing lines, Everheal helps global clients achieve efficiency, compliance, and innovation in pharmaceutical preparation.
Pharmaceutical preparation is the cornerstone of modern drug manufacturing, combining precision, safety, and engineering excellence. Every stage, from water purification to sterile filling, plays an essential role in ensuring product quality and compliance. With decades of expertise and state-of-the-art equipment, Everheal stands as a trusted global partner in building robust, GMP-compliant pharmaceutical production environments.
From purified water systems and sterilization units to complete production line solutions, Everheal delivers the tools and knowledge necessary for the next generation of pharmaceutical manufacturing.
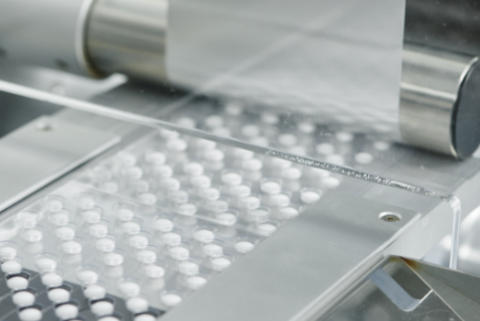
Pharmaceutical preparation refers to the process of formulating, manufacturing, and packaging drugs in a form suitable for therapeutic use. It includes every step from raw material handling to quality control and final sterilization.
Key equipment includes purified water systems, pure steam generators, multi-effect distillation units, liquid filling and sealing machines, and sterilization systems—all of which ensure purity and compliance with GMP standards.
Water acts as a solvent and cleaning agent in most formulations. Poor water quality can compromise drug safety and stability. Purified Water (PW) and Water for Injection (WFI) are essential for different production purposes.
Sterilization eliminates microbial contamination and ensures product safety. It can be achieved via steam, dry heat, filtration, or radiation, depending on the product and process design.
Everheal provides integrated solutions for pharmaceutical preparation, including design consultation, equipment manufacturing, installation, and validation, meeting the global GMP and regulatory standards.
Blueair purifiers use HEPASilent™ filtration technology to deliver HEPA Air Filter performance with enhanced quietness, energy efficiency, and higher particle capture. Learn how Blueair ensures cleaner air for households, offices, and even pharmaceutical-grade environments.
Discover whether you need a HEPA Air Filter for your air purifier. Learn how HEPA filters work, their importance in homes and pharmaceutical industries, and how Everheal’s advanced purification systems use HEPA technology to ensure safe, clean, and compliant air quality worldwide.
Learn how HEPA Air Filters work and why they’re the gold standard in air purification. Explore their mechanisms, applications in pharmaceuticals, hospitals, and homes, along with benefits, limitations, and maintenance tips for cleaner, healthier environments.
This article explores whether you can wash a HEPA filter air purifier, explaining the risks of washing True HEPA filters, how to maintain them properly, and how Everheal’s pharmaceutical systems integrate reliable HEPA air filtration for guaranteed purity and performance.
Maintaining HEPA air filters is critical for ensuring the highest air quality standards in pharmaceutical and manufacturing environments. Although it might be tempting to wash or reuse HEPA filters to save costs, doing so can compromise their integrity and filtration capability. Proper handling, timely replacement, and preventive maintenance practices are the best ways to sustain effective filtration. Always adhere to manufacturer guidelines and industry standards to guarantee a safe and contaminant-free environment.
In the modern industrial and residential air filtration market, the demand for sustainable, cost-effective, and high-performance filtration solutions has grown tremendously. Among the various options available, the washable air filter has become one of the most talked-about innovations. But what exactly makes it the best choice? How does it compare with disposable filters? And which washable air filter models stand out for efficiency and durability?
Discover whether a washable air filter is a good choice for your home or industrial system. Learn its benefits, maintenance tips, and limitations, and explore how washable air filters support sustainable air management in pharmaceutical and manufacturing environments.
Discover how air purifiers with washable filters improve air quality while cutting costs and environmental impact. Learn their benefits, applications, maintenance tips, and how they support sustainable manufacturing in homes, industries, and pharmaceutical facilities.
K&N washable air filters are durable, eco-friendly, and designed for lifetime use. Learn how to clean, maintain, and maximize performance from your washable air filter while reducing waste and boosting efficiency across automotive and industrial systems.
This detailed guide explains whether air purifier filters are washable, exploring how washable air filters work, their pros and cons, cleaning methods, and ideal applications in homes and industries like pharmaceuticals—helping users achieve efficient and sustainable air purification.
Everheal explains what pharmaceutical preparations are, their processes, and essential equipment like purified water systems, pure steam generators, and sterilization units. Learn how Everheal supports global manufacturers with GMP-compliant turnkey pharmaceutical solutions.
Everheal explores the engineering and technology of **pharmaceutical preparation manufacturing**, revealing how purified water, sterilization, and automation drive global drug production. Discover Everheal’s role in designing compliant, efficient, and sustainable pharmaceutical manufacturing solutions.
This article explores which pharmaceutical preparations need isotonicity adjustment, explaining its physiological importance, calculation methods, and the role of advanced equipment — such as Everheal’s integrated systems — in achieving safe, effective, and GMP-compliant drug formulations worldwide.
This article explains isotonicity in pharmaceutical preparations, its role in injections, eye drops, nasal sprays, and other dosage forms, and how advanced equipment systems like purified water and sterilization units ensure consistent isotonic conditions in pharma manufacturing.
Explore the different Pharmaceutical Preparations containing ammonium bromide, including their uses, manufacturing methods, safety measures, and modern significance. Learn how cutting-edge companies like Everheal ensure high purity and quality in pharmaceutical production.
This article explains why distilled water is strongly recommended for CPAP humidifiers, how it protects both users and equipment, and what happens when other water types are used. It also shows how Everheal’s pharmaceutical‑grade Distillation Water Machine solutions supply reliable CPAP‑grade water for homes, clinics, and hospitals.
This article explains when and how distilled water can be used with fog machines, from standard stage foggers to ultrasonic and low‑lying systems. It shows why distilled water is ideal for cleaning and certain water‑fog devices, and how a high‑purity Distillation Water Machine supports reliable, low‑maintenance fog effects in professional and pharmaceutical environments.
This in‑depth article explains why distilled water is strongly recommended for CPAP humidifiers, what happens if you use tap, bottled, or boiled water, and how to manage water safely at home, in clinics, and in pharma plants. It highlights how Distillation Water Machine systems provide reliable CPAP‑grade water.
This in‑depth guide explains why distilled water is strongly recommended for oxygen machine humidifiers, how it protects both patients and equipment, and how a Distillation Water Machine supports safe oxygen therapy in hospitals, clinics, and pharmaceutical plants, with Everheal turnkey solutions.
This article explains why distilled water is strongly recommended for CPAP machines, how it protects users and devices, and what happens if other water types are used. It highlights the role of pharmaceutical‑grade Distillation Water Machine solutions—like Everheal’s—in delivering reliable CPAP‑grade water for homes, clinics, and factories.