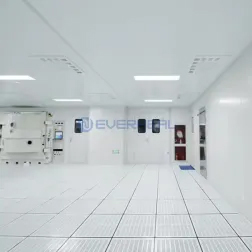
EVERHEAL pharmatech clean room decoration mainly includes: all kinds of steel sandwich panel for walls and ceiling, clean room use floor , clean room use doors and windows, airshower, interlocks, clean room telephone, installation of ventilation pipes, wind valves, air filtering system(HEPA), HVAC system, air exhausting system, power supply, electrical control system, alarm system, thunder-arresting and electrical ground system, cooling equipment etc. It also includes: air conditioner in the centre of testing center, air purifying of aseptic testing rooms and raw material sampling room.
ISO 14644-1 Clean Room Standards |
Class | Maximum Particles/m3 | Federal Standard 209E |
≥0.1 µm | ≥0.2 µm | ≥0.3 µm | ≥0.5 µm | ≥1 µm | ≥5 µm | Equivalent |
ISO 1 | 10 | 2 |
|
|
|
|
|
|
ISO 2 | 100 | 24 | 10 | 4 |
|
|
|
|
ISO 3 | 1,000 | 237 | 102 | 35 | 8 |
| Class 1 |
|
ISO 4 | 10,000 | 2,370 | 1,020 | 352 | 83 |
| Class 10 |
|
ISO 5 | 100,000 | 23,700 | 10,200 | 3,520 | 832 | 29 | Class 100 |
ISO 6 | 1,000,000 | 237,000 | 102,000 | 35,200 | 8,320 | 293 | Class 1,000 |
ISO 7 |
|
|
| 352,000 | 83,200 | 2,930 | Class 10,000 |
|
ISO 8 |
|
|
| 3,520,000 | 832,000 | 29,300 | Class 100,000 |
|
ISO 9 |
|
|
| 35,200,000 | 8,320,000 | 293,000 | Room Air |
|
In the rapidly evolving field of pharmaceutical manufacturing, maintaining a clean and controlled environment is of utmost importance. To meet the industry's stringent regulations and ensure the highest level of product quality, the introduction of the
In the rapidly evolving field of pharmaceutical manufacturing, maintaining a clean and controlled environment is of utmost importance. To meet the industry's stringent regulations and ensure the highest level of product quality, the introduction of the Pharmaceutical Cleanroom System Portable GMP Modular is a game-changer. This state-of-the-art solution revolutionizes cleanroom technology, providing pharmaceutical manufacturers with unparalleled flexibility, efficiency, and compliance.
At its core, the Portable GMP Modular Cleanroom System is designed to create a controlled environment that minimizes contamination and optimizes the production process. It offers a modular construction, allowing for easy installation, reconfiguration, and expansion without the need for extensive construction work or downtime. This unique flexibility allows pharmaceutical companies to adapt to changing production demands quickly, effortlessly expanding or modifying their cleanroom operations as needed.
One of the remarkable features of this cleanroom system is its Compliance with Good Manufacturing Practices (GMP). As pharmaceutical manufacturing is heavily regulated, GMP compliance is obligatory. The Portable GMP Modular Cleanroom System ensures that all GMP guidelines are effectively adhered to, providing an assurance of quality and safety for both the producer and the end consumer. It incorporates specialized filtration systems, robust air handling units, and reliable monitoring systems, all of which work cohesively to maintain a controlled environment free from contaminants.
The Portable GMP Modular Cleanroom System also excels in energy efficiency. By utilizing advanced energy-saving technologies, it enables pharmaceutical manufacturers to reduce their energy consumption significantly while still maintaining the required air quality standards. This not only contributes to a more sustainable manufacturing process but also results in substantial cost savings in the long run.
Furthermore, this cleanroom system is equipped with cutting-edge monitoring and control systems. These systems continuously monitor critical parameters such as temperature, humidity, air pressure differentials, and particle count, ensuring that the cleanroom environment remains within specified limits. Real-time data is collected and analyzed, allowing for immediate identification of potential issues or deviations from the desired conditions. This proactive approach enables prompt corrective actions, minimizing risks and enhancing overall operational efficiency.
Another notable advantage of the Pharmaceutical Cleanroom System Portable GMP Modular is its seamless integration with industry-standard equipment and technologies. This cleanroom system can be easily tailored to accommodate various manufacturing processes and equipment, including filling machines, isolators, compounding equipment, and more. As a result, pharmaceutical manufacturers can optimize their workflow within a controlled environment, reducing the risk of cross-contamination and product degradation.
In terms of maintenance and cleaning, the Portable GMP Modular Cleanroom System offers unparalleled ease of use. The modular design facilitates efficient cleaning and decontamination processes, while the removable panels and fixtures allow for easy access to every corner of the cleanroom. Additionally, the system is built using materials that are resistant to corrosion and microbial growth, ensuring the longevity and hygienic properties of the cleanroom infrastructure.
In summary, the Pharmaceutical Cleanroom System Portable GMP Modular is a revolutionary solution that sets a new standard in pharmaceutical manufacturing. Its modular construction, GMP compliance, energy efficiency, advanced monitoring systems, and seamless integration capabilities make it an unrivaled choice for pharmaceutical companies seeking a cleanroom system that can adapt to their evolving needs. With this innovative solution, manufacturers can achieve exceptional levels of cleanliness, efficiency, and ultimately, deliver safe and high-quality pharmaceutical products to consumers.
Mainly Applications
Hot Tags: Pharmaceutical Cleanroom System, Clean Room Equipment, Pharmaceutical Clean Room, Clean Room In Pharmaceutical Industry, Clean Room In Pharma Industry, Clean Room Pharmaceutical, Pharma Clean Room Equipment, Clean Room Standards, Cleanrooms In Pharmaceutical Industry, Pharmaceutical Industry Equipment, China, Customized, OEM, Low price, manufacturing company, manufacturers, factory, suppliers, quotation, for sale