Views: 222 Author: Rebecca Publish Time: 2025-12-10 Origin: Site











Content Menu
● Understanding Isotonicity in Pharmaceutical Preparations
>> The Role of Isotonicity in Drug Formulation
● Mechanisms for Achieving Isotonicity
● Importance of Isotonicity Across Pharmaceutical Preparations
>> 1. Parenteral Preparations (Injectables)
>> 2. Ophthalmic Preparations (Eye Drops & Solutions)
● Industrial Role of Isotonicity in Pharmaceutical Facility Design
>> Key Systems Supporting Isotonicity Control
● Methods of Testing Isotonicity
● Challenges and Future Trends
● FAQs About Isotonicity in Pharmaceutical Preparations
>> 1. What does isotonicity mean in pharmaceutical preparation?
>> 2. Why is isotonicity important in injections?
>> 3. Which pharmaceutical preparations require isotonicity?
>> 4. How can isotonicity be achieved?
>> 5. What equipment supports isotonicity control in manufacturing?
In the pharmaceutical industry, isotonicity plays a crucial role in developing safe and effective pharmaceutical preparations. It ensures that solutions administered to the human body do not disrupt cellular balance, minimize irritation, and promote patient comfort. Understanding isotonicity is particularly important for injectable, ophthalmic, and nasal formulations, as deviations from isotonic conditions can cause adverse physiological reactions.
This article explores in detail what isotonicity means, why it matters in pharmaceutical preparation, how it is achieved, and which types of pharmaceutical preparations require isotonicity. It also connects these scientific fundamentals to the realm of industrial manufacturing — such as water purification systems, sterilization equipment, and filling machines — which ensure isotonic solutions are safely and consistently produced.

Isotonicity refers to the condition where a solution has the same osmotic pressure as body fluids like blood plasma or tears. In simpler terms, an isotonic solution maintains the same concentration of solute particles as the fluids of the cells it comes into contact with.
In pharmaceutical preparation, maintaining isotonicity prevents unwanted osmotic movement of water across cell membranes. If a solution is not isotonic, it can either cause cells to shrink (in hypertonic solutions) or swell and burst (in hypotonic solutions).
For this reason, isotonicity is essential in drug formulations that interact directly with sensitive body tissues.
The maintenance of isotonicity in pharmaceutical preparations ensures:
- Preserved tissue integrity.
- Minimized pain upon administration.
- Increased stability of the drug solution.
- Enhanced patient compliance due to reduced irritation.
Such formulations commonly include injectable drugs, ophthalmic products, nasal drops, and irrigating solutions — all of which have a direct physiological interface.
In the process of pharmaceutical preparation, isotonicity is achieved through the adjustment of solute concentrations. Common isotonic agents include sodium chloride, dextrose, and mannitol.
Two major laboratory methods are typically employed:
1. Freezing Point Depression Method – Based on the principle that the freezing point of body fluids is approximately -0.52°C. Adjusting a formulation to this freezing point ensures isotonicity.
2. Sodium Chloride Equivalent Method (E-Value) – Calculates the amount of NaCl or other solutes required to achieve isotonic concentration in a preparation.
These methods are largely performed using purified water prepared by pharmaceutical water systems, such as those produced by Everheal, which ensures endotoxin-free, high-purity water for consistent isotonic formulations.
Injectable pharmaceutical preparations are among the most critical forms requiring isotonicity. When a parenteral solution is injected directly into the bloodstream or tissues, its osmotic pressure must match that of blood plasma to prevent red blood cell deformation and vascular irritation.
Examples include:
- Intravenous infusions (e.g., 0.9% sodium chloride).
- Intramuscular injections.
- Subcutaneous injections.
Hypertonic injections can cause pain and thrombophlebitis, while hypotonic injections can cause hemolysis. Manufacturers use sterile purified water systems, multi-effect distillation water machines, and pure steam generators to ensure the precise preparation of such isotonic parenteral products.
The eye is highly sensitive to osmotic pressure variations. Hence, isotonicity is crucial in pharmaceutical preparations like eye drops, ointments, or irrigating solutions.
Solutions intended for ophthalmic use must be isotonic with tears (roughly 0.9% NaCl). Deviations can cause stinging, tearing, or blurred vision. Common isotonic agents used in these preparations include sodium chloride, potassium chloride, and boric acid.
Manufacturing impact: Automation systems, filling machines, and sterile filtration are used to ensure the precise concentration and sterility of isotonic ophthalmic preparations.
Nasal formulations are another class of pharmaceutical preparations that demand isotonic conditions. The nasal mucosa is extremely vascularized, allowing drugs to quickly enter systemic circulation.
If a nasal solution is hypertonic, it can lead to mucosal dehydration and discomfort; if hypotonic, it may cause swelling and congestion. Maintaining isotonicity ensures optimal absorption and comfort.
Irrigating solutions used during surgical or diagnostic procedures must also be isotonic to prevent irritation or tissue damage. These pharmaceutical preparations are employed in washing wounds, body cavities, or tissues.
For example:
- Normal saline is used as an isotonic irrigant in surgery.
- Isotonic buffer systems are employed in cleansing procedures.
Because these are used in sensitive environments, they are sterilized using autoclaves or sterilizing systems similar to those offered by Everheal, ensuring complete microbial safety without altering osmotic balance.
Inhalation solutions, such as nebulized medications, should ideally be isotonic with respiratory tract secretions, maintaining comfort and avoiding bronchial irritation.
Isotonicity in these pharmaceutical preparations ensures:
- Better mucociliary clearance.
- Reduced coughing reflex.
- Stable aerosol particle formation.

Establishing isotonic pharmaceutical products consistently requires advanced manufacturing equipment and controlled environmental systems. Everheal, as a professional supplier of pharmaceutical equipment, plays a key role in enabling isotonicity control through its integrated production solutions.
- Purified Water Preparation Systems: Ensure constant supply of high-purity water for isotonic dissolution.
- Pure Steam Generators: Provide sterile steam for equipment sanitization, ensuring contamination-free production.
- Multi-Effect Distillation Machines: Produce Water for Injection (WFI) critical for isotonic injectable formulations.
- Liquid Filling and Sealing Machines: Enable accurate dosing and prevent variation in solution concentration.
- Sterilization Systems: Maintain aseptic standards crucial for sensitive isotonic pharmaceutical preparations.
Through custom factory layout planning and production line design, these systems help pharmaceutical manufacturers meet GMP and international regulatory standards while ensuring product isotonicity consistency.
Maintaining isotonicity requires precise analytical control during pharmaceutical preparation. Common tests include:
- Hemolytic Method: Checks the impact of the solution on red blood cells.
- Freezing Point Determination: Compares the freezing point of the formulation with body fluids.
- Coligative Property Measurements: Evaluate osmotic pressure or vapor pressure lowering.
Advanced pharmaceutical laboratories use automated measurement systems and quality control protocols to ensure isotonic balance in every batch.
Maintaining isotonicity is not only a formulation challenge but also a manufacturing one. As biopharmaceuticals, vaccines, and nanomedicines become more complex, the importance of isotonicity will grow.
Future trends involve:
- Real-time isotonicity monitoring using digital sensors.
- Integration of AI in pharmaceutical preparation control systems.
- Automated adjustment of osmotic pressure during batch production.
Innovations like these will reshape how pharmaceutical companies ensure isotonic stability while maintaining production efficiency and regulatory compliance.
Isotonicity stands as a foundational requirement in modern pharmaceutical preparation, essential for ensuring safety, efficacy, and comfort across a variety of dosage forms — especially injectables, ophthalmic, nasal, and irrigating solutions.
Maintaining isotonicity demands not only scientific expertise but also advanced equipment such as purified water systems, distillation units, and sterilization facilities. Companies like Everheal provide pharmaceutical manufacturers with the integrated technological solutions necessary for producing isotonic formulations that comply with global GMP standards.
In the evolving pharmaceutical landscape, achieving isotonicity consistently is both a scientific necessity and a manufacturing benchmark for quality assurance.
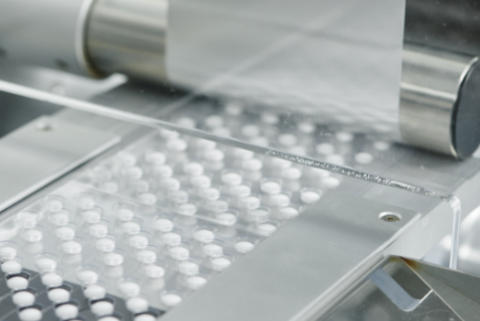
Isotonicity refers to a solution's osmotic pressure being equal to that of body fluids. In pharmaceutical preparation, isotonicity ensures the solution does not cause cell rupture or irritation when administered.
Because injectable pharmaceutical preparations come in direct contact with body fluids, maintaining isotonicity prevents hemolysis, pain, and tissue damage.
Injectables, ophthalmic solutions, nasal sprays, and irrigating solutions are the main categories requiring isotonicity. These interact directly with sensitive biological tissues.
It is typically achieved by adding isotonic agents like sodium chloride or dextrose and verifying osmotic pressure using freezing point or E-value calculations during pharmaceutical preparation.
Purified water systems, sterilization machines, multi-effect distillers, and filling lines ensure consistent isotonic conditions in pharmaceutical production facilities.
Blueair purifiers use HEPASilent™ filtration technology to deliver HEPA Air Filter performance with enhanced quietness, energy efficiency, and higher particle capture. Learn how Blueair ensures cleaner air for households, offices, and even pharmaceutical-grade environments.
Discover whether you need a HEPA Air Filter for your air purifier. Learn how HEPA filters work, their importance in homes and pharmaceutical industries, and how Everheal’s advanced purification systems use HEPA technology to ensure safe, clean, and compliant air quality worldwide.
Learn how HEPA Air Filters work and why they’re the gold standard in air purification. Explore their mechanisms, applications in pharmaceuticals, hospitals, and homes, along with benefits, limitations, and maintenance tips for cleaner, healthier environments.
This article explores whether you can wash a HEPA filter air purifier, explaining the risks of washing True HEPA filters, how to maintain them properly, and how Everheal’s pharmaceutical systems integrate reliable HEPA air filtration for guaranteed purity and performance.
Maintaining HEPA air filters is critical for ensuring the highest air quality standards in pharmaceutical and manufacturing environments. Although it might be tempting to wash or reuse HEPA filters to save costs, doing so can compromise their integrity and filtration capability. Proper handling, timely replacement, and preventive maintenance practices are the best ways to sustain effective filtration. Always adhere to manufacturer guidelines and industry standards to guarantee a safe and contaminant-free environment.
In the modern industrial and residential air filtration market, the demand for sustainable, cost-effective, and high-performance filtration solutions has grown tremendously. Among the various options available, the washable air filter has become one of the most talked-about innovations. But what exactly makes it the best choice? How does it compare with disposable filters? And which washable air filter models stand out for efficiency and durability?
Discover whether a washable air filter is a good choice for your home or industrial system. Learn its benefits, maintenance tips, and limitations, and explore how washable air filters support sustainable air management in pharmaceutical and manufacturing environments.
Discover how air purifiers with washable filters improve air quality while cutting costs and environmental impact. Learn their benefits, applications, maintenance tips, and how they support sustainable manufacturing in homes, industries, and pharmaceutical facilities.
K&N washable air filters are durable, eco-friendly, and designed for lifetime use. Learn how to clean, maintain, and maximize performance from your washable air filter while reducing waste and boosting efficiency across automotive and industrial systems.
This detailed guide explains whether air purifier filters are washable, exploring how washable air filters work, their pros and cons, cleaning methods, and ideal applications in homes and industries like pharmaceuticals—helping users achieve efficient and sustainable air purification.
Everheal explains what pharmaceutical preparations are, their processes, and essential equipment like purified water systems, pure steam generators, and sterilization units. Learn how Everheal supports global manufacturers with GMP-compliant turnkey pharmaceutical solutions.
Everheal explores the engineering and technology of **pharmaceutical preparation manufacturing**, revealing how purified water, sterilization, and automation drive global drug production. Discover Everheal’s role in designing compliant, efficient, and sustainable pharmaceutical manufacturing solutions.
This article explores which pharmaceutical preparations need isotonicity adjustment, explaining its physiological importance, calculation methods, and the role of advanced equipment — such as Everheal’s integrated systems — in achieving safe, effective, and GMP-compliant drug formulations worldwide.
This article explains isotonicity in pharmaceutical preparations, its role in injections, eye drops, nasal sprays, and other dosage forms, and how advanced equipment systems like purified water and sterilization units ensure consistent isotonic conditions in pharma manufacturing.
Explore the different Pharmaceutical Preparations containing ammonium bromide, including their uses, manufacturing methods, safety measures, and modern significance. Learn how cutting-edge companies like Everheal ensure high purity and quality in pharmaceutical production.
This article explains why distilled water is strongly recommended for CPAP humidifiers, how it protects both users and equipment, and what happens when other water types are used. It also shows how Everheal’s pharmaceutical‑grade Distillation Water Machine solutions supply reliable CPAP‑grade water for homes, clinics, and hospitals.
This article explains when and how distilled water can be used with fog machines, from standard stage foggers to ultrasonic and low‑lying systems. It shows why distilled water is ideal for cleaning and certain water‑fog devices, and how a high‑purity Distillation Water Machine supports reliable, low‑maintenance fog effects in professional and pharmaceutical environments.
This in‑depth article explains why distilled water is strongly recommended for CPAP humidifiers, what happens if you use tap, bottled, or boiled water, and how to manage water safely at home, in clinics, and in pharma plants. It highlights how Distillation Water Machine systems provide reliable CPAP‑grade water.
This in‑depth guide explains why distilled water is strongly recommended for oxygen machine humidifiers, how it protects both patients and equipment, and how a Distillation Water Machine supports safe oxygen therapy in hospitals, clinics, and pharmaceutical plants, with Everheal turnkey solutions.
This article explains why distilled water is strongly recommended for CPAP machines, how it protects users and devices, and what happens if other water types are used. It highlights the role of pharmaceutical‑grade Distillation Water Machine solutions—like Everheal’s—in delivering reliable CPAP‑grade water for homes, clinics, and factories.